CEO and Co-founder of the Center for Contemporary Sciences, Dr. Aysha Akhtar M.D., M.P.H., was interviewed by Dr. Erika Gebel Berg Ph.D., Director and Science Editor of the Science Custom Publishing Group. This podcast interview was originally broadcasted and transcribed by the Science Podcast. The conversation included context around the historic achievement of the FDA Modernization Act 2.0 passed in 2022, the downside to animal testing, and the relevance of human biology-based methodologies for drug research and development.
The Center for Contemporary Sciences TL;DR
📃 The FDA Modernization Act 2.0 is a historic achievement in that the law passed on a unanimous vote across the political spectrum, highlighting the recognition on both sides of the aisle for the need to shift toward human-relevant R&D methodologies
💉 Despite conventional animal testing, more than 9 out of 10 drugs and vaccines that cross animal tests end up failing when tried in humans. It’s the differences between human biology and animal biology that are problematic for drug and therapeutics R&D
❌ There are deeply entrenched cultural barriers that still exist within the scientific fields and within scientific journals as well that prevent the accelerated adoption of human-relevant methodologies
Complete Podcast Transcript
Erica Berg: Hello to our listeners, and welcome to the sponsored interview from the Science AAAS Custom Publishing Office, and brought to you by Michelson Philanthropies. I’m Erica Berg, director and Senior Editor for Custom Publishing at Science. Today I am delighted to welcome Dr. Aysha Akhtar, a leader in the fields of animal ethics and neurology. We’ll be having a conversation about how a recent law and advances in technology could someday soon allow scientists to develop a new drug without lab rats. Thank you so much for joining Dr. Akhtar.
Aysha Akhtar: Thanks so much for having me here, Erica.
EB: Before we get started, I wanted to share that I recently had the chance to catch up with Dr. Gary Michelson about how this new law came to pass. In addition to the Michelson Medical Research Foundation, he established the Michelson Center for Public Policy, which advocates for increased funding for biomedical research equity in higher education and animal cruelty prevention working with federal officials. He helped secure the passage of the FDA Modernization Act 2.0, which was signed into law in 2022. Here’s what Dr. Michelson had to say about this historic achievement.
“What made this particular piece of legislation truly remarkable was that you had Rand Paul and Cory Booker, who are sort of the bookends on the political spectrum coming together in recognition of how important this was. And then I think the more remarkable part that nobody believes when I tell them is it actually passed on a unanimous vote. When you tell anybody it was a unanimous vote in that year, they go, “No, it never happened.”
EB: It’s always nice when government works, isn’t it? Aysha, my first question for you is about the FDA Modernization Act 2.0. My question is, what does that law modernize, and why?
AA: Currently the Food and Drug Administration or the FDA requires that all new drugs and vaccines have to be tested on two animal species before the FDA allows them to move on to be tested in human clinical trials. And so that was actually a regulation that evolved from a law called the Federal Food Drug and Cosmetic Act of 1938. The FFDCA of 1938 was in response to a couple of problems that happened. There was a sulfa drug, an antibiotic drug that the company wanted to make it sweeter, so it was more palatable for children. In order to make it sweeter, what the company did was they add ethylene glycol, which is antifreeze that led to more than a hundred deaths, many of them, or most of them in children. Basically, the sulfa drug situation led to this requirement that all new drugs and vaccines have to be tested on animals before they can be tried in humans and approved for the market. So the Food and Drug Modernization Act 2.0 basically updates that depression-era law by saying, yeah, if you want to do animal testing, you can, but you can also use other more cutting edge, more innovative, and more modern techniques in place of animal testing if you want. So it catches up the law to the science.
EB: From a purely scientific perspective, what are the downsides of using animal models to stand in for humans?
AA:
We now know that despite the animal testing, more than 9 out of 10 drugs and vaccines that cross animal tests end up failing when tried in humans. There are many things that we share with the biology of a rat and a dog and a non-human primate, including chimpanzees, but it’s the differences between our biology and the biology of these other animals that are causing the problem.
EB: I’m gonna switch gears a little bit now to talk about the alternatives. I’ve done a bit of reading about the technologies that are currently in development as replacements for animal models, and they sound like they’re all straight out of science fiction. Can you tell me about the leading animal replacement technologies? Let’s start with organoids.
AA: Sure. I know they sound so fascinating, and if you look at them at the images and the videos of them, they’re quite cool. Organoids are basically miniature organs that you grow in the lab using, for example, adult stem cells and they mimic the physiology of that actual organ. They mimic the function, the biology of that actual organ. You can use them also to test whether a drug is going to be safe in that organ and whether a drug will work in that organ.
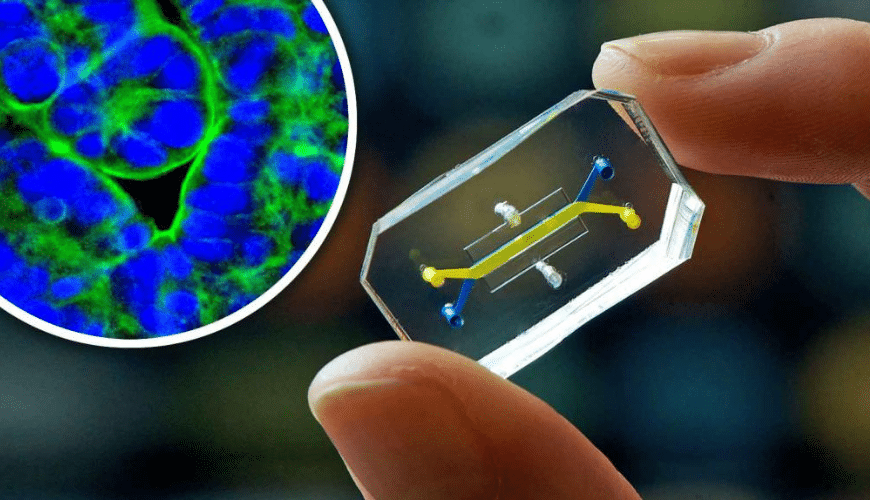
EB: Another technology I read about is called organ on a chip or animal on a chip or human on a chip. What can you tell us about those?
AA: Right. So the organ on a chip goes even further than the organoids because it’s in a setting that mimics the actual setting of the organ in the human body. So a lung on a chip actually breathes and functions like a major part of the actual lung in human body.
So one day what’s gonna happen, Erica, is that a researcher is going to be able to take my cells and create my organs on a chip and integrate them to create an Aysha body on a chip. You’re gonna be able to take your cells and create an Erica on a chip so that they can use your body on a chip to actually screen drugs to see if they’re actually going to be safe in your body, and if they’re actually gonna work against any of the diseases you may experience.
EB: Erica on a chip someday. Okay. [chuckle]
EB: So the other bucket of technologies I was reading about sort of away from the wet lab and into a computer. What can you tell me about how far we’ve come and the uses of virtual cells or virtual organs, virtual animals? Where are we with those technologies and how do they work?
AA: You can call it a digital twin or a virtual patient. And a virtual patient basically is capturing data from multiple sources, which could be population data, clinical trial data, electronic health records, and basically it combines multiple different types of testing methods and data sources.
EB: What is the idea for how these technologies would actually be incorporated into the drug development process to replace animal models?
AA: So right now many of these techniques are being used in the drug development process, but sort of alongside animal testing, which quite honestly doesn’t make sense because these other methods can so far outperform the animal tests and be such better predictors of human outcomes than the other tests. I think what’s gonna happen is that as the field becomes more and more comfortable and confident and experienced with using these newer methods, eventually they will replace the use of animals. Totally. And they will be used to predict whether a drug will be safe and effective in humans. As a matter of fact, we can actually use these technologies one day to perform human clinical trials that are in the lab.
EB: What are the hurdles that are preventing scientists from using these technologies to replace animal and I guess human trials as well?
AA: Regulatory agencies still require animal testing. So even though that law, the FDA Modernization Act does not require animal testing for drug development, it still leaves the decision-making ultimately at the hands of the Food and Drug Administration.
There’s regulatory barriers and there’s funding barriers. There are also publication barriers. I’ve heard from numerous scientists that are working on these more advanced technologies that when they try to publish their results, they get kickback from the scientific publication saying, “No, we want to also see these results in animal experiments.” So there’s this entrenched cultural barrier that exists within the scientific fields and within scientific journals as well. So all of those barriers still need to be removed.
EB: Aysha, are there success stories from other industries that you can talk about and how they reduced their dependence on animal testing?
AA: Yeah Erica, that’s a great question. So many times people say, oh, but we cannot replace animal testing. Well, that’s what the cosmetic industry used to say 20, 30 years ago. It took the work of advocates, animal protection advocate organizations that forced the cosmetic industry to change. Animal testing is hardly done now for cosmetics.
EB: Aysha, is there anything else you’d like to share with us today? We’re running low on time, but I just wanted to give you one more opportunity to share insights with us.
AA: I think Erica just thank you so much for having this conversation, for talking about such a needed topic. I would just add that I’m really truly excited about where we’re headed in medical science.
EB: Aysha, thank you so much for taking the time to talk with me today. These are indeed exciting times to live and to hope that the next big life-saving drug may not need to come at the expense of animals. To learn more about the work that Dr. Akhtar and her colleagues are doing, please visit contemporarysciences.org. Our thanks to Michelson Philanthropies for making this conversation possible. And thank you to Chris Connor for audio support. And finally, thank you, dear listener, for spending this time with us.