2023 Year In Review

Top 5 Innovators to Watch in 2024
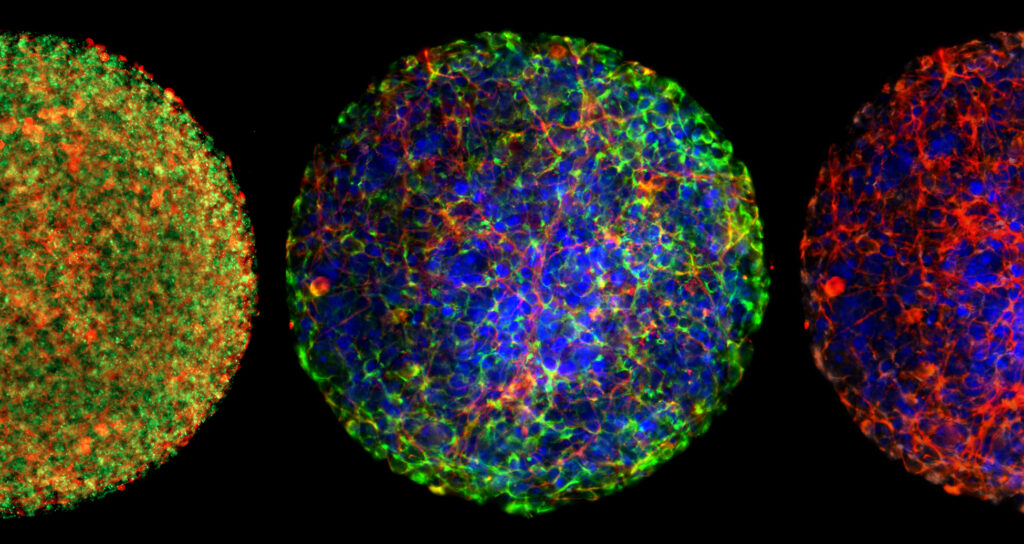
Why Organ-Chips are Better at Modeling Human Diseases than Animal Testing
Updates on Human-Relevant Methods in Pharmaceutical Medicine and the Legislative Process
On December 1, 2022, the Center for Contemporary Sciences (CCS) hosted its second Discovery Forum. The event was an opportunity to discuss the updates on human-relevant methods that can replace animal testing and the FDA Modernization Act 2.0. Speakers across industry and academia met to give their takeaways on how to improve the drug development […]
Animals in Laboratories Speak

Every year, more than 100 million animals are used in experimentation in the U.S. The Animal Welfare Act (AWA) is the only U.S. federal statute that regulates the treatment of animals used in experiments. However, it excludes >95% of animals used. Additionally, even for the species which are covered, the AWA provides no limits on […]
CCS Investor Summit 2022 Report

On March 24, 2022, CCS hosted its first ever Investor Summit in partnership with Beyond Animal. The Summit was open to the public and brought together an international group of innovators, investors, researchers, start-ups, and venture philanthropists. The packed, three-hour-Summit was an opportunity to provide needed exposure to business entities in the space, promote the […]
2021 Year in Review

The Future of Parkinson’s Disease Research

Check out CCS’s latest scientific publication on Parkinson’s Disease research. In coordination with the Center for Alternatives to Animal Testing (CAAT), CCS held a workshop May 2021 to develop a scheme that outlines the basic research and testing approaches necessary to make PD research a success—elucidating pathological mechanisms, identifying knowledge gaps, discovering druggable targets, and developing […]
2020 Annual Report
Recent Advances in the Development and Application of Human-Specific Biomedical Research and Testing Methods